What should I consider when conveying toxic and explosive powders?
When moving powder from process to process in the pharmaceutical and fine chemical industries, several crucial factors must be addressed to ensure an acceptable solution. The method of moving powders in an enclosed stream of air or nitrogen below ambient pressure is commonly known as vacuum conveying and the technology for this form of transport has evolved to meet the arduous demands of these markets. Typically there are seven key goals which the end user, in conjunction with the equipment supplier, should consider when addressing the use of vacuum conveying. The article "Seven key parameters to consider when conveying toxic and explosive powders" addresses these seven key parameters which must be considered when conveying toxic and explosive powders.
How can we pneumatically convey our cohesive pharmaceutical powders more effectively?
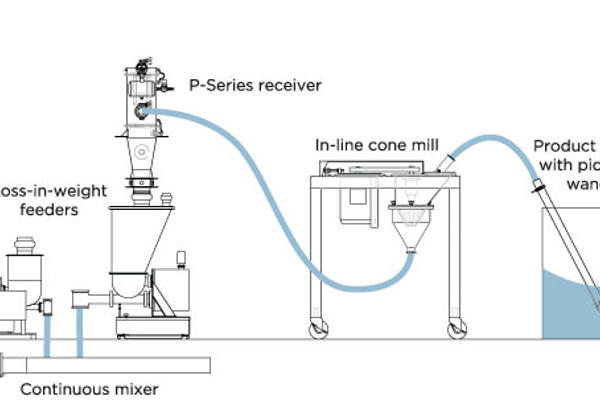
Pharmaceutical powders often include very poor flowing excipients and API’s (Active pharmaceutical ingredients) which can present challenges not only in material handling but also in areas such as product and process containment, options in design for cleaning and product changeover. Pneumatic conveying operations can often be incorporated directly into the secondary process in an effort to ensure a closed and leak-free operation, without any exposure of operator or product. These additional processes include, but are not limited to: direct blender loading, inline conical screen milling or sieving, tablet press loading, granulator loading/unloading, fluid bed dryer unloading, tablet/capsule conveying, refill of screw feeders for loading of continuous processes such as granulation, mixing, milling/micronization, and extrusion, transfer direct to feeders for batch weighing or dispensing of several components in a blend. A number of key criteria should be evaluated when discussing your application with the pneumatic system vendor and choosing the proper pneumatic conveying technology including the following:
- Properly define the material characteristics: Proper evaluation of a pneumatic conveying system for a cohesive, slightly sticky material requires you to define your material's characteristics, such as bulk density, flowability, particle size distribution, moisture content, abrasiveness, and temperature. These characteristics will all affect not only the proper technology to be used, (e.g. dense phase vacuum versus dilute phase vacuum), but will also affect overall system geometries and design, such as angles on receiver and pickup hoppers and additional flow aid requirements.
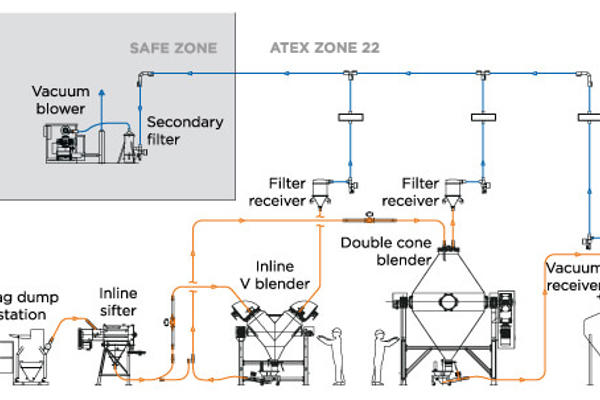
- Define the overall conveying requirements: These definitions will include a general description of your application and purpose, the system's location and environment classification, the conveying rate, the horizontal conveying distance, the vertical conveying distance, conveying line direction changes (bends), a material source description, and a material destination description. It will also include details on the material explosive properties (which may require an inert conveying gas medium) as well as the toxicity of the powder, which will define the levels of containment required not only during the material transfer but also at both the product pickup and discharge points.
- If possible, perform bench material testing: If the material can be sent for testing, be sure to send a small representative material sample to a well-equipped pneumatic conveying system supplier for bench testing. If the material is too toxic for testing, then sending a placebo with similar flow and material characteristics is also acceptable. The supplier will issue a test report to confirm and complete the material characteristics testing. This test report, along with the conveying requirements provided in the second step, will help determine the best theoretical pneumatic conveying system configuration.
Each of the criteria described above will enable the most efficient choice for pneumatic transfer. For example, in the case of designing a system for a slightly cohesive material, the optimal pneumatic conveying system configuration may consist of the following:
- A dilute-phase pneumatic conveying system, which is the most likely system type as it is the most forgiving, given the application.
- A motive gas supply with a positive-displacement blower package or a Venturi for low rate and small space applications.
- A line charger consisting of a blow-through rotary airlock with a nonstick coating and shear protection.
- A conveying line that is properly supported and sized with flexible rubber hose and flexible rubber hose bends.
- A motive gas and material separation filter, which has a small pleated cartridge filter media with a nonstick coating and is easy to take apart and clean.
In all cases of material handling, it is important to discuss in detail all of the items above with an equipment system supplier who is well versed in the properties of a wide variety of pharmaceutical powders. This will ensure not only the most efficient means of transfer, but also the safest means of transfer for both operator and final product.